1. Introduction
Rayon is the oldest fibre, is the regenerated cellulose fibre with a wide spectrum properties. Cellulose is to be one of the most useable natural polymers worldwide. It is biodegradable & renewable polymer. The common source for industrial purpose are wood pulp and cotton lint. Highly purified wood pulp consists of 95 – 99% cellulose. It is called ‘chemical cellulose’ & ‘dissolving pulp’. Those chemical cellulose or dissolving pulps are use to manufacture man-made fibres (e.g. viscose rayon, cellulose acetate). The process used to make viscose can either be a continuous or batch process. The batch process is flexible in producing a wide variety of rayons having broad Rayon's versatility is the result of the fibre being chemically and structurally engineered by making use of the properties of cellulose from which it is made. However, it is somewhat difficult to control uniformity between batches and it also requires high labour involvement. The continuous process is the main method for producing rayon. Three methods of production lead to distinctly different types of rayon fibres, viscose rayon, cuprammonium rayon and saponified cellulose acetate. |
Method of dissolving cellulose were first discovered in the late 19th century and first fibre were made by dissolving cellulose in cuprammoniumm hydroxide and then forcing the solution through tinny orifice into a bath containing reagent to remove solvent to regenerate cellulose in filament form. Problem associated with lack of stability and considerations of cost competitiveness soon pushed this method into background. With the discovery of the cellulose the cellulosic have been and still are predominantly produced by this process. Due to the strong intermolecular bonds, cellulose does not melt and does not dissolve readily in ordinarily available solvents; chemists have resorted to the derivatization of cellulose to render it soluble and process-able. Specifically, the viscose process was developed. It converted cellulose into sodium cellulose xanthate, which was soluble in a caustic solution, making it possible to wet-spin the polymer into a fibre or film. This technique was accepted worldwide and has prospered. The process, however, consists of multiple steps and causes pollution. As a result, end users have looked for alternate methods of processing cellulose.
2. Different types of rayons
Rayon fibres are engineered to possess a range of properties to meet the demands for a wide variety of end uses. Some of the important types of fibres are briefly described-
a. High wet modulus yarn- These fibres have exceptionally high wet modulus of about 1 g/den and are used as parachute cords and other industrial uses. Fortisan fibres made by Celanese (saponified acetate) has also been used for the same purpose.
b. Polynosic rayon-These fibres have a very high degree of orientation, achieved as a result of very high stretching (up to 300 %) during processing. They have a unique fibrillar structure, high dry and wet strength, low elongation (8 to 11 %), relatively low water retention and very high wet modulus.
3. Specialty rayons
3.1 Flame retardant fibres- Flame retardance is achieved by the adhesion of the correct flame- retardant chemical to viscose. Examples of additives are alkyl, aryl and halogenated alkyl or aryl phosphates, phosphazenes, phosphonates and polyphosphonates. Flame retardant rayons have the additives distributed uniformly through the interior of the fibre and this property is advantageous over flame retardant cotton fibres where the flame retardant concentrates at the surface of the fibre.
3.2 Super absorbent rayons-This is being produced in order to obtain higher water retention capacity (although regular rayon retains as much as 100 % of its weight). These fibres are used in surgical nonwovens. These fibres are obtained by including water- holding polymers (such as sodium polyacrylate or sodium carboxy methyl cellulose) in the viscose prior to spinning, to get a water retention capacity in the range of 150 to 200 % of its weight.
3.3 Micro denier fibres- rayon fibres with deniers below 1.0 are now being developed and introduced into the market. These can be used to substantially improve fabric strength and absorbent properties.
3.4 Cross section modification- Modification in cross sectional shape of viscose rayon can be used to dramatically change the fibres' aesthetic and technical properties. One such product is Viloft, a flat cross sectional fibre sold in Europe, which gives a unique soft handle, pleasing drape and handle. Another modified cross section fibre called Fibre ML(multi limbed) has a very well defined trilobal shape. Fabrics made of these fibre have considerably enhanced absorbency, bulk, cover and wet rigidity all of which are suitable for usage as nonwovens [10].
3.5 Tencel rayon-Unlike viscose rayon, Tencel is produced by a straight solvation process. Wood pulp is dissolved in an amine oxide, which does not lead to undue degradation of the cellulose chains. The clear viscous solution is filtered and extruded into an aqueous bath, which precipitates the cellulose as fibre. This process does not involve any direct chemical reaction and the diluted amine oxide is purified and reused. This makes for a completely contained process fully compatible with all environmental regulations.
3.6 Lyocell- A new form of cellulosic fibre, Lyocell, is starting to find uses in the nonwovens industry. Lyocell is manufactured using a solvent spinning process, and is produced by only two companies -- Acordis and Lenzing AG. To produce Lyocell, wood cellulose is dissolved directly in n-methyl morpholine n-oxide at high temperature and pressure. The cellulose precipitates in fibre form as the solvent is diluted, and can then be purified and dried. The solvent is recovered and reused. Lyocell has all the advantages of rayon, and in many respects is superior. It has high strength in both dry and wet states, high absorbency, and can fibrillate under certain conditions. In addition, the closed-loop manufacturing process is far more environmentally friendly than that used to manufacture rayon, although it is also more costly.
Uses-Some major uses in apparel like as shirts, blouses, blankets, window treatment dresses, jackets, hats ,socks, bedsheets & industrial uses such as tire cord, non- woven product,& also medical surgery product and other uses as hygiene product ,diapers, towels. Rayon is the major feedstock in the production of carbon fibre.
4. Manufacturing Process of Viscose Rayon Fiber
a. High wet modulus yarn- These fibres have exceptionally high wet modulus of about 1 g/den and are used as parachute cords and other industrial uses. Fortisan fibres made by Celanese (saponified acetate) has also been used for the same purpose.
b. Polynosic rayon-These fibres have a very high degree of orientation, achieved as a result of very high stretching (up to 300 %) during processing. They have a unique fibrillar structure, high dry and wet strength, low elongation (8 to 11 %), relatively low water retention and very high wet modulus.
3. Specialty rayons
3.1 Flame retardant fibres- Flame retardance is achieved by the adhesion of the correct flame- retardant chemical to viscose. Examples of additives are alkyl, aryl and halogenated alkyl or aryl phosphates, phosphazenes, phosphonates and polyphosphonates. Flame retardant rayons have the additives distributed uniformly through the interior of the fibre and this property is advantageous over flame retardant cotton fibres where the flame retardant concentrates at the surface of the fibre.
3.2 Super absorbent rayons-This is being produced in order to obtain higher water retention capacity (although regular rayon retains as much as 100 % of its weight). These fibres are used in surgical nonwovens. These fibres are obtained by including water- holding polymers (such as sodium polyacrylate or sodium carboxy methyl cellulose) in the viscose prior to spinning, to get a water retention capacity in the range of 150 to 200 % of its weight.
3.3 Micro denier fibres- rayon fibres with deniers below 1.0 are now being developed and introduced into the market. These can be used to substantially improve fabric strength and absorbent properties.
3.4 Cross section modification- Modification in cross sectional shape of viscose rayon can be used to dramatically change the fibres' aesthetic and technical properties. One such product is Viloft, a flat cross sectional fibre sold in Europe, which gives a unique soft handle, pleasing drape and handle. Another modified cross section fibre called Fibre ML(multi limbed) has a very well defined trilobal shape. Fabrics made of these fibre have considerably enhanced absorbency, bulk, cover and wet rigidity all of which are suitable for usage as nonwovens [10].
3.5 Tencel rayon-Unlike viscose rayon, Tencel is produced by a straight solvation process. Wood pulp is dissolved in an amine oxide, which does not lead to undue degradation of the cellulose chains. The clear viscous solution is filtered and extruded into an aqueous bath, which precipitates the cellulose as fibre. This process does not involve any direct chemical reaction and the diluted amine oxide is purified and reused. This makes for a completely contained process fully compatible with all environmental regulations.
3.6 Lyocell- A new form of cellulosic fibre, Lyocell, is starting to find uses in the nonwovens industry. Lyocell is manufactured using a solvent spinning process, and is produced by only two companies -- Acordis and Lenzing AG. To produce Lyocell, wood cellulose is dissolved directly in n-methyl morpholine n-oxide at high temperature and pressure. The cellulose precipitates in fibre form as the solvent is diluted, and can then be purified and dried. The solvent is recovered and reused. Lyocell has all the advantages of rayon, and in many respects is superior. It has high strength in both dry and wet states, high absorbency, and can fibrillate under certain conditions. In addition, the closed-loop manufacturing process is far more environmentally friendly than that used to manufacture rayon, although it is also more costly.
Uses-Some major uses in apparel like as shirts, blouses, blankets, window treatment dresses, jackets, hats ,socks, bedsheets & industrial uses such as tire cord, non- woven product,& also medical surgery product and other uses as hygiene product ,diapers, towels. Rayon is the major feedstock in the production of carbon fibre.
4. Manufacturing Process of Viscose Rayon Fiber
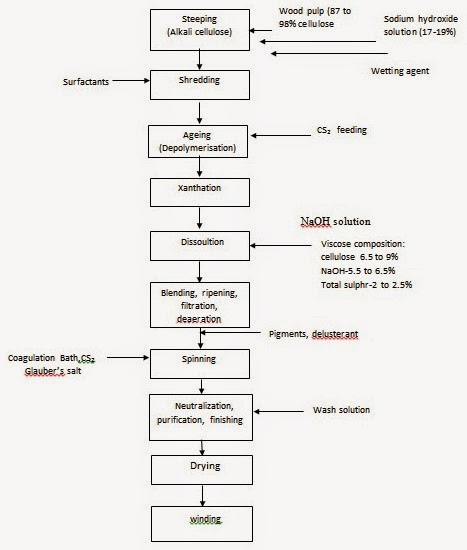
The process of manufacturing viscose rayon consists of the following steps mentioned, in the order that they are carried out: (1) Steeping, (2) Pressing, (3) Shredding, (4) Aging, (5) Xanthation, (6) Dissolving, (7)Ripening, (8) Filtering, (9) Degassing, (10) Spinning, (11) Drawing, (12) Washing, (13) Cutting. The various steps involved in the process of manufacturing viscose are shown in Fig. and clarified below.
(1) Steeping: Cellulose pulp is immersed in 17-20% aqueous sodium hydroxide (NaOH) at a temperature in the range of 18 to 25°C in order to swell the cellulose fibres and to convert cellulose to alkali cellulose.
(C6H10O5)n + nNaOH ---> (C6H9O4ONa)n + nH2O
(2) Pressing: The swollen alkali cellulose mass is pressed to a wet weight equivalent of 2.5 to 3.0 times the original pulp weight to obtain an accurate ratio of alkali to cellulose.
(3) Shredding: The pressed alkali cellulose is shredded mechanically to yield finely divided, fluffy particles called "crumbs". This step provides increased surface area of the alkali cellulose, thereby increasing its ability to react in the steps that follow.
(4) Aging: The alkali cellulose is aged under controlled conditions of time and temperature (between 18 and 30° C) in order to depolymerize the cellulose to the desired degree of polymerization. In this step the average molecular weight of the original pulp is reduced by a factor of two to three. Reduction of the cellulose is done to get a viscose solution of right viscosity and cellulose concentration.
(5) Xanthation: In this step the aged alkali cellulose crumbs are placed in vats and are allowed to react with carbon disulphide under controlled temperature (20 to 30°C) to form cellulose xanthate.
(C6H9O4ONa)n + nCS2 ----> (C6H9O4O-SC-SNa)n
Side reactions that occur along with the conversion of alkali cellulose to cellulose xanthate are responsible for the orange colour of the xanthate crumb and also the resulting viscose solution. The orange cellulose xanthate crumb is dissolved in dilute sodium hydroxide at 15 to 20 °C under high-shear mixing conditions to obtain a viscous orange coloured solution called "viscose", which is the basis for the manufacturing process. The viscose solution is then filtered (to get out the insoluble fibre material) and is deaerated.
(6) Dissolving: The yellow crumb is dissolved in aqueous caustic solution. The large xanthate substituents on the cellulose force the chains apart, reducing the inter-chain hydrogen bonds and allowing water molecules to solvate and separate the chains, leading to solution of the otherwise insoluble cellulose. Because of the blocks of un-xanthated cellulose in the crystalline regions, the yellow crumb is not completely soluble at this stage. Because the cellulose xanthate solution (or more accurately, suspension) has a very high viscosity, it has been termed "viscose".
(7) Ripening: The viscose is allowed to stand for a period of time to "ripen". Two important process occur during ripening: Redistribution and loss of xanthate groups. The reversible xanthation reaction allows some of the xanthate groups to revert to cellulosic hydroxyls and free CS2. This free CS2 can then escape or react with other hydroxyl on other portions of the cellulose chain. In this way, the ordered, or crystalline, regions are gradually broken down and more complete solution is achieved. The CS2 that is lost reduces the solubility of the cellulose and facilitates regeneration of the cellulose after it is formed into a filament.
(C6H9O4O-SC-SNa)n + nH2O ---> (C6H10O5)n + nCS2 + nNaOH
(8) Filtering: The viscose is filtered to remove undissolved materials that might disrupt the spinning process or cause defects in the rayon filament.
(9) Degassing: Bubbles of air entrapped in the viscose must be removed prior to extrusion or they would cause voids, or weak spots, in the fine rayon filaments.
(10) Spinning - (Wet Spinning): Production of Viscose Rayon Filament: The viscose solution is metered through a spinnerette into a spin bath containing sulphuric acid (necessary to acidify the sodium cellulose xanthate), sodium sulphate (necessary to impart a high salt content to the bath which is useful in rapid coagulation of viscose), and zinc sulphate (exchange with sodium xanthate to form zinc xanthate, to cross link the cellulose molecules). Once the cellulose xanthate is neutralized and acidified, rapid coagulation of the rayon filaments occurs which is followed by simultaneous stretching and decomposition of cellulose xanthate to regenerated cellulose. Stretching and decomposition are vital for getting the desired tenacity and other properties of rayon. Slow regeneration of cellulose and stretching of rayon will lead to greater areas of crystallinity within the fibre, as is done with high-tenacity rayons.
The dilute sulphuric acid decomposes the xanthate and regenerates cellulose by the process of wet spinning. The outer portion of the xanthate is decomposed in the acid bath, forming a cellulose skin on the fibre. Sodium and zinc sulphates control the rate of decomposition (of cellulose xanthate to cellulose) and fibre formation.
(C6H9O4O-SC-SNa)n + (n/2)H2SO4 --> (C6H10O5)n + nCS2 + (n/2)Na2SO4
Elongation-at-break is seen to decrease with an increase in the degree of crystallinity and orientation of rayon.
In standard viscose of 30-50 poise viscosity made with 32% CS2 is spun into an aqueous acid salt spin bath of the following type at a temperature of 40-50 0c .
(11) Drawing: The rayon filaments are stretched while the cellulose chains are still relatively mobile. This causes the chains to stretch out and orient along the fibre axis. As the chains become more parallel, inter-chain hydrogen bonds form, giving the filaments the properties necessary for use as textile fibres.
(12) Washing: The freshly regenerated rayon contains many salts and other water soluble impurities which need to be removed. Several different washing techniques may be used.
(13) Cutting: If the rayon is to be used as staple (i.e., discreet lengths of fibre), the group of filaments (termed "tow") is passed through a rotary cutter to provide a fibre which can be processed in much the same way as cotton.
5. Structure of Rayon
In regenerated celluloses, the unit cell structure is an allotropic modification of cellulose I, designated as cellulose II (other allotropic modifications are also known as cellulose III and cellulose IV). The structure of cellulose derivatives could be represented by a continuous range of states of local molecular order rather than definite polymorphic forms of cellulose which depend on the conditions by which the fibre is made. Rayon fibre properties will depend on: how cellulose molecules are arranged and held together; the average size and size distribution of the molecules.
 |
| Manufacturing of Viscose Rayon |
(C6H10O5)n + nNaOH ---> (C6H9O4ONa)n + nH2O
(2) Pressing: The swollen alkali cellulose mass is pressed to a wet weight equivalent of 2.5 to 3.0 times the original pulp weight to obtain an accurate ratio of alkali to cellulose.
(3) Shredding: The pressed alkali cellulose is shredded mechanically to yield finely divided, fluffy particles called "crumbs". This step provides increased surface area of the alkali cellulose, thereby increasing its ability to react in the steps that follow.
(4) Aging: The alkali cellulose is aged under controlled conditions of time and temperature (between 18 and 30° C) in order to depolymerize the cellulose to the desired degree of polymerization. In this step the average molecular weight of the original pulp is reduced by a factor of two to three. Reduction of the cellulose is done to get a viscose solution of right viscosity and cellulose concentration.
(5) Xanthation: In this step the aged alkali cellulose crumbs are placed in vats and are allowed to react with carbon disulphide under controlled temperature (20 to 30°C) to form cellulose xanthate.
(C6H9O4ONa)n + nCS2 ----> (C6H9O4O-SC-SNa)n
Side reactions that occur along with the conversion of alkali cellulose to cellulose xanthate are responsible for the orange colour of the xanthate crumb and also the resulting viscose solution. The orange cellulose xanthate crumb is dissolved in dilute sodium hydroxide at 15 to 20 °C under high-shear mixing conditions to obtain a viscous orange coloured solution called "viscose", which is the basis for the manufacturing process. The viscose solution is then filtered (to get out the insoluble fibre material) and is deaerated.
(6) Dissolving: The yellow crumb is dissolved in aqueous caustic solution. The large xanthate substituents on the cellulose force the chains apart, reducing the inter-chain hydrogen bonds and allowing water molecules to solvate and separate the chains, leading to solution of the otherwise insoluble cellulose. Because of the blocks of un-xanthated cellulose in the crystalline regions, the yellow crumb is not completely soluble at this stage. Because the cellulose xanthate solution (or more accurately, suspension) has a very high viscosity, it has been termed "viscose".
(7) Ripening: The viscose is allowed to stand for a period of time to "ripen". Two important process occur during ripening: Redistribution and loss of xanthate groups. The reversible xanthation reaction allows some of the xanthate groups to revert to cellulosic hydroxyls and free CS2. This free CS2 can then escape or react with other hydroxyl on other portions of the cellulose chain. In this way, the ordered, or crystalline, regions are gradually broken down and more complete solution is achieved. The CS2 that is lost reduces the solubility of the cellulose and facilitates regeneration of the cellulose after it is formed into a filament.
(C6H9O4O-SC-SNa)n + nH2O ---> (C6H10O5)n + nCS2 + nNaOH
(8) Filtering: The viscose is filtered to remove undissolved materials that might disrupt the spinning process or cause defects in the rayon filament.
(9) Degassing: Bubbles of air entrapped in the viscose must be removed prior to extrusion or they would cause voids, or weak spots, in the fine rayon filaments.
(10) Spinning - (Wet Spinning): Production of Viscose Rayon Filament: The viscose solution is metered through a spinnerette into a spin bath containing sulphuric acid (necessary to acidify the sodium cellulose xanthate), sodium sulphate (necessary to impart a high salt content to the bath which is useful in rapid coagulation of viscose), and zinc sulphate (exchange with sodium xanthate to form zinc xanthate, to cross link the cellulose molecules). Once the cellulose xanthate is neutralized and acidified, rapid coagulation of the rayon filaments occurs which is followed by simultaneous stretching and decomposition of cellulose xanthate to regenerated cellulose. Stretching and decomposition are vital for getting the desired tenacity and other properties of rayon. Slow regeneration of cellulose and stretching of rayon will lead to greater areas of crystallinity within the fibre, as is done with high-tenacity rayons.
The dilute sulphuric acid decomposes the xanthate and regenerates cellulose by the process of wet spinning. The outer portion of the xanthate is decomposed in the acid bath, forming a cellulose skin on the fibre. Sodium and zinc sulphates control the rate of decomposition (of cellulose xanthate to cellulose) and fibre formation.
(C6H9O4O-SC-SNa)n + (n/2)H2SO4 --> (C6H10O5)n + nCS2 + (n/2)Na2SO4
Elongation-at-break is seen to decrease with an increase in the degree of crystallinity and orientation of rayon.
In standard viscose of 30-50 poise viscosity made with 32% CS2 is spun into an aqueous acid salt spin bath of the following type at a temperature of 40-50 0c .
- H2SO4-8-10%
- Na2SO4-16-24%
- ZnSO4-1-2%
(11) Drawing: The rayon filaments are stretched while the cellulose chains are still relatively mobile. This causes the chains to stretch out and orient along the fibre axis. As the chains become more parallel, inter-chain hydrogen bonds form, giving the filaments the properties necessary for use as textile fibres.
(12) Washing: The freshly regenerated rayon contains many salts and other water soluble impurities which need to be removed. Several different washing techniques may be used.
(13) Cutting: If the rayon is to be used as staple (i.e., discreet lengths of fibre), the group of filaments (termed "tow") is passed through a rotary cutter to provide a fibre which can be processed in much the same way as cotton.
5. Structure of Rayon
 |
| Figure 1: Structure of cellulose structure |
- Cellulose I is shown in native cellulose.
- Cellulose II has been seen in regenerated cellulose or and mercerized cellulose.
- Cellulose III is produce when treat with liquid ammonia (NH3) or organic amines (RNH2).
- Cellulose IV is generate when we treat cellulose with heat and glycerol (CH2(OH)CH(OH)CH2(OH)).
 |
Many models describe ways in which the cellulose molecules may be arranged to form fibre fine structure. The most popular models of fibre fine structure are the fringed micelle and fringed fibrillar structures. Essentially, they all entail the formation of crystallites or ordered regions.
The skin-core effect is very prominent in rayon fibres. Mass transfer in wet spinning is a slow process (which accounts for the skin-core effect) compared to the heat transfer in melt spinning. The skin contains numerous small crystallites and the core has fewer but larger crystallites. The skin is stronger and less extensible, compared to the core. It also swells less than the core; hence, water retention is lower in the skin than in the core although moisture regain is higher in the skin. This is explained by an increased number of hydroxyl groups available for bonding with water as a result of a larger total surface area of the numerous small crystallites.
When rayon fibres are worked in the wet state,the filament structure can be made to disintegrate into a fibrillar texture. The extent to which this occurs reflects the order that exists in the fibre structure, as a consequence of the way in which the cellulose molecules are brought together in spinning. Another important structural feature of rayon fibre is its cross-sectional shape. Various shapes include round, irregular, Y-shaped, E-shaped, U-shaped, T-shaped and flat.
6. Properties of Rayon
The skin-core effect is very prominent in rayon fibres. Mass transfer in wet spinning is a slow process (which accounts for the skin-core effect) compared to the heat transfer in melt spinning. The skin contains numerous small crystallites and the core has fewer but larger crystallites. The skin is stronger and less extensible, compared to the core. It also swells less than the core; hence, water retention is lower in the skin than in the core although moisture regain is higher in the skin. This is explained by an increased number of hydroxyl groups available for bonding with water as a result of a larger total surface area of the numerous small crystallites.
 |
| Fig. 5: Cellulose structure |
6. Properties of Rayon
Variations during spinning of viscose or during drawing of filaments provide a wide variety of fibres with a wide variety of properties. These include:
In the non-zinc spinning process under normal conditions of acid concentrated in the spinning bath ,cellulose xanthate gel is converted into cellulose xanthic acid and then to cellulose .The process is very fast and regeneration takes place before the cellulose molecules can properly oriented .This result in a rather disorientation matrix with poor crystalline organization .The net result is a regenerated cellulose filament with poor dry strength and a very interior wet strength. So the zinc result in a transient zinc cellulose xanthate complex which is more stable against acid induced regeneration. Zinc being bivalent form a transient cross link between the adjustment xanthate groups. Coupled with crosslinkg the strong deswelling action of zinc xanthate gel which can be stretched to a highly oriented structure with small crystal size and relatively large crystals.
8. Spinning with Modifiers
It should be recognized that in the presence of zinc, modifiers enhance the action of zinc in spinning bath. It do not effect the viscose. Its mechanism is the formation of a semipermeable membrane by the combined action of zinc ions by the product trithiocarbamate ions from the viscose and the modifiers .This semipermeable retards the diffusion of both Zn+ and H+ ions in the filament ,but actual ratio Zn+ to H+ ion penetration is markedly increased in the presence of modifiers. Hense its act as barrier for proton diffusion .Hense its acidification boundary shift further away from the spinning nozzle in the presence of modifiers.
Types of modifiers
A viscose solution of viscosity 100 poise containing modifiers 1-3% by weight of cellulose and with a CS2 content of 40% is spun underripe (salt index-6-15) into a aqueous spinning bath containing –
8.2 Modified high wet-modulus yarns
The condition of viscose solution and spinning bath composition are generally similar to those tyre yarns.
Spinning bath temperature-350c is kept lower because it gives more deformable gel necessitating a slower spinning speed 20-40 m/min The result is that gel fibres are stretched at an earlier state of the gel dehydration and decomposition when the gel is more plastic and can be stretched more(125%-150%).
8.3 Polynosic fibre
Polynosic is similar rayon fibre but difference in process of manufacturing than viscose rayon. Since the manufacturing process is different so their morphological structure also different. Generally polynosic fibre has high crystallinity and high orientation. This give high mechanical strength and chemical resistant, high wet modulus and more dimension stable.
NOTE- In polynosic process we eliminated the Ageing stage, Ripening stage ,Diluted acid concentration & zinc sulphate.
Viscose solution:
8.4 Super high wet modulus rayon
By adding 1% formaldehyde to spin bath or to the viscose substantially increases the toughness and plasticity of viscose gel. We can get the stretch of 500-600% .Disadvantage of this compound is that it is very toxic.
9. Fibre variant for improved bulk & handle
Approaches has been to produce high performance crimped fibres where the bulk is due to the interaction between the fibres, creating bulk in resultant yarns and fabric. The second approach has been to produce an inherently bulky fibre using an inflation technique during fibre production.
Composition for producing high wet performance fibres,
9.1 High performance crimped fibres
For many years standard crimped fibres have been available which are produced by altering the regeneration condition so that the skin of the fibre burst while still in spin bath. The liquid viscose thus processed is regenerated under slightly different conditions and a bicomponent structure result. The two parts of the fibre shrink differently in subsequently washing and drying processes and the fibre develops a permanent crimp as a result.
9.2 Inflated fibres
In this a range of fibres cross-section can be produced ,but a tubular structure provide the best combination of bulk and handle while still retaining the physical properties and processing performance of standard viscose rayon.
9.3 Spinning specifications
9.4 Super absorbent fibres
These fibres are used in sanitary protection and in surgical dressing. For these end uses high absorbency and purity. For these fibre highly hydrophilic polymers that are also compatible with viscose are added to the spinning solution. Chemicals such as sodium polyacrylate, carboxymetyl cellulose,acrylamide-2-metylpropne sulphonic acid.
9.5 Flame retardant fibres
Flame retardant additives are added in the viscose dope. Some of compound used are halogenized triaryl ,halogenized alkyl phosphate ,halogenized alkyl thiophosphate, aloxyphosphopanzenes. A flame retardant fibre using a flame retardant compound is phosphorous. The flame retardant compound is mixed with viscose solution prior to spinning.
Limiting oxygen index (LOI) of more than 26% qualifies a material for flame retardency, Tufban’s LOI value of 30-32% makes it an excellent fire resistant material. It is claimed that normal washing and dry –cleaning do not effect its flame retardant characteristics.
10. Incorpation of carbon in viscose fiber
(a) Incorporation of carbon black for antistatic properties-Electrically conductive carbon is used for the production of electrically conductive viscose fibre. The conductivity increases with decreasing particle size of carbon. For the production of electrically conductive viscose fibres, a slightly alkaline , electric conductive carbon black with a particle size of 20 nm is dispersed in water and mixed in viscose solution prior to spinning. It is found that a loss of about 50% in tenacity of this fibre compared with the tenacity of regular viscose fibre. On the other hand electricity conductive is increased by five orders of magnitude.
(b) Incorporation of activated carbon-Activated carbon containing fibre with outstanding adsorption properties. For the production of activated carbon-containing viscose fibres, activated carbon from coconut with a very fine pores is used .This activated carbon is has to be aground to a very fine particle size and thoroughly dispersed in water in order to be incorporated in viscose fibre in a satisfactory way. These fibres are used in application in such as protective clothing against gases, flat structure for industrial gas adsorportion, shoe insoles odour-absorbing and antimicrobial wound dressing.
(c) Incorporation of graphite-Incorporating 40% of lubricating graphite with a purity 99.5% into viscose yields fibres with excellent lubricating properties which as packing and sealing for crankshafts. Packings from graphite containing viscose fibres may be used to a temperature of 180-2000c.They are stable in the Ph range 5-9 and predominantly used to seal pumps and fitting for water, salt solutions, weak organic and inorganic acids.
11. Alternative to the viscose process
The viscose process provides inexpensive route to regenerate cellulose in fibrous form it constituent a health hazard due to toxic pollutants. Two major route which are much easier and less polluting than the old viscose process.
1. In which dissolution involves nearly simultaneous derivative formation for example metal-amine solvents, N2O4-DMF,DMSO-PF SYSTEM.
2. That produces a derivative from which cellulose can be easily generated for example-liquid ammonia salt system.
Organic solvent (Direct solvent)
1. Ammonia-ammonium thiocyanate- The solvent is prepared by condensing NH3 to a predetermined weight with a known amount of NH4SCN. Solution of cellulose in NH3-NH4SCN are prepared by making a slurry of cellulose and solvent and stirring at- 10 0c.Fibres have been spun using wet spinning ,dry spinning and dry-jet wet spinning containing 14% cellulose at a solvent composition of 24.5:75.5 (wt%) NH3:NH4SCN
2. N-Methylmorpholine N-Oxide and Water-
3. N-N Dimethyl acetamide and lithium chloride
Solution process
Salt that melt at temp. below 1000c called ionic liquid. It can be used as green solvent. Or reaction media. Generally contain imidazolium, pyridinium or organic ammonium salt. The anions could be chloride, bromide. Room temp. have more complex structure .The polymer can be regenerated by precipitation with water. They are able to achieve a very high polymer concentration of up to 25%.
5. Amine salt
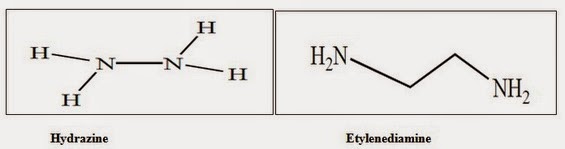
Two component system (amine salt) consisting of hydrazine (NH2-NH2) or ethylenediamine (NH2-CH2-CH2-NH2) and various thiocyanate salt such as LISCN,NASCN or KSCN that dissolve cellulose pump. However a high concentration 40-50% . Salt is generally required to obtain high concentration (upto 18-20% w/w) spinning dope.
Hydrazine is a good solvent swelling agent, similar to ammonia . Its boiling point 113.50c which is much higher than (-33.40c ) .So that due to high boiling point its offer same potential advantages for investigating the solubility and behaviour of cellulose.
12. Development in process technology
Difficulties
Reduction in chemical used such as CS2,NaOH and H2SO4. There are number of ways to achieve reduction in the amount of CS2 used in viscose & also allow a substantial reduction in viscose alkali content. Process-SINI process-Its also known as double steeping process operation of aged alkali cellulose at lower alkali concentration (10-12%).A second steeping after ages reduces the amount of free alkali in the crumb with out changing the bound alkali. This reduces the formation of by-product and improves distribution of xanthate group to get a stable viscose.
Advantages
13. Conclusion
- Fibres with thickness of 1.7 to 5.0dtex, particularly those between 1.7 and 3.3 dtex, dominate large scale production.
- Tenacity ranges between 2.0 to 2.6 g/den when dry and 1.0 to 1.5 g/den when wet.
- Wet strength of the fibre is of importance during its manufacturing and also in subsequent usage. Modifications in the production process have led to the problem of low wet strength being overcome.
- Dry and wet tenacity extend over a range depending on the degree of polymerization and crystallinity. The higher the crystallinity and orientation of rayon, the lower is the drop in tenacity upon wetting.
- Percentage elongation-at-break seems to vary from 10 to 30 % dry and 15 to 40 % wet. Elongation-at-break is seen to decrease with an increase in the degree of crystallinity and orientation of rayon.
- Thermal properties: Viscose rayon loses strength above 149°C; chars and decomposes at 177 to 204°C. It does not melt or stick at elevated temperatures.
- Chemical properties: Hot dilute acids attack rayon, whereas bases do not seem to significantly attack rayon. Rayon is attacked by bleaches at very high concentrations and by mildew under severe hot and moist conditions. Prolonged exposure to sunlight causes loss of strength because of degradation of cellulose chains.
- Abrasion resistance is fair and rayon resists pill formation. Rayon has both poor crease recovery and crease retention.
In the non-zinc spinning process under normal conditions of acid concentrated in the spinning bath ,cellulose xanthate gel is converted into cellulose xanthic acid and then to cellulose .The process is very fast and regeneration takes place before the cellulose molecules can properly oriented .This result in a rather disorientation matrix with poor crystalline organization .The net result is a regenerated cellulose filament with poor dry strength and a very interior wet strength. So the zinc result in a transient zinc cellulose xanthate complex which is more stable against acid induced regeneration. Zinc being bivalent form a transient cross link between the adjustment xanthate groups. Coupled with crosslinkg the strong deswelling action of zinc xanthate gel which can be stretched to a highly oriented structure with small crystal size and relatively large crystals.
 |
It should be recognized that in the presence of zinc, modifiers enhance the action of zinc in spinning bath. It do not effect the viscose. Its mechanism is the formation of a semipermeable membrane by the combined action of zinc ions by the product trithiocarbamate ions from the viscose and the modifiers .This semipermeable retards the diffusion of both Zn+ and H+ ions in the filament ,but actual ratio Zn+ to H+ ion penetration is markedly increased in the presence of modifiers. Hense its act as barrier for proton diffusion .Hense its acidification boundary shift further away from the spinning nozzle in the presence of modifiers.
Types of modifiers
- Tertiary amine
- Quaternary ammonium salt
- Polyoxyalkylene derivative
- Polyoxyhydroxy polyamide
- Dithiocarbamates
A viscose solution of viscosity 100 poise containing modifiers 1-3% by weight of cellulose and with a CS2 content of 40% is spun underripe (salt index-6-15) into a aqueous spinning bath containing –
- H2SO4 8-10%
- Na2SO4 16-24%
- ZnSO4 6%
8.2 Modified high wet-modulus yarns
The condition of viscose solution and spinning bath composition are generally similar to those tyre yarns.
Spinning bath temperature-350c is kept lower because it gives more deformable gel necessitating a slower spinning speed 20-40 m/min The result is that gel fibres are stretched at an earlier state of the gel dehydration and decomposition when the gel is more plastic and can be stretched more(125%-150%).
8.3 Polynosic fibre
Polynosic is similar rayon fibre but difference in process of manufacturing than viscose rayon. Since the manufacturing process is different so their morphological structure also different. Generally polynosic fibre has high crystallinity and high orientation. This give high mechanical strength and chemical resistant, high wet modulus and more dimension stable.
NOTE- In polynosic process we eliminated the Ageing stage, Ripening stage ,Diluted acid concentration & zinc sulphate.
Viscose solution:
- 6% cellulose
- 4.4% NaOH
- 500-600 D.P
- 500 poise viscosity
- H2SO4-2-3%
- Na2SO4-4-6%
- Temp-250c
- Spinning speed-20-30m/min
- Stretch-150-300%
Crystallinity(%) | Birefringence | |
Standard Viscose | 45.2 | 0.027 |
Tyre yarn | 41.5 | 0.037 |
Polynosic | 55.2 | 0.046 |
8.4 Super high wet modulus rayon
By adding 1% formaldehyde to spin bath or to the viscose substantially increases the toughness and plasticity of viscose gel. We can get the stretch of 500-600% .Disadvantage of this compound is that it is very toxic.
9. Fibre variant for improved bulk & handle
Approaches has been to produce high performance crimped fibres where the bulk is due to the interaction between the fibres, creating bulk in resultant yarns and fabric. The second approach has been to produce an inherently bulky fibre using an inflation technique during fibre production.
Composition for producing high wet performance fibres,
Cellulose | 7-7.5 |
NaOH | 6-7.5 |
CS2 | 30-32 |
Modifiers: Dimetylamine | .8-1.5 |
Polyethylene glycol | .8-1.5 |
9.1 High performance crimped fibres
For many years standard crimped fibres have been available which are produced by altering the regeneration condition so that the skin of the fibre burst while still in spin bath. The liquid viscose thus processed is regenerated under slightly different conditions and a bicomponent structure result. The two parts of the fibre shrink differently in subsequently washing and drying processes and the fibre develops a permanent crimp as a result.
9.2 Inflated fibres
In this a range of fibres cross-section can be produced ,but a tubular structure provide the best combination of bulk and handle while still retaining the physical properties and processing performance of standard viscose rayon.
9.3 Spinning specifications
Xanthate sulphur (%) | .8-1.2 |
Viscosity (poise) | 80-100 |
Salt index(NaCl) | 4-6 |
DP(fibre) | 400-600 |
Primary Bath | Secondary Bath | |
H2SO4 | 5-5.5 | 2.5-4.5 |
Na2SO4 | 15 | |
ZnSO4(%) | 2-3 | |
Temp.(0c) | 35-45 | 95+ |
Stretch(%) | 90-100 | 25-30 |
Take-up speed(m/min) | 30-50 | 30-50 |
9.4 Super absorbent fibres
These fibres are used in sanitary protection and in surgical dressing. For these end uses high absorbency and purity. For these fibre highly hydrophilic polymers that are also compatible with viscose are added to the spinning solution. Chemicals such as sodium polyacrylate, carboxymetyl cellulose,acrylamide-2-metylpropne sulphonic acid.
9.5 Flame retardant fibres
Flame retardant additives are added in the viscose dope. Some of compound used are halogenized triaryl ,halogenized alkyl phosphate ,halogenized alkyl thiophosphate, aloxyphosphopanzenes. A flame retardant fibre using a flame retardant compound is phosphorous. The flame retardant compound is mixed with viscose solution prior to spinning.
Limiting oxygen index (LOI) of more than 26% qualifies a material for flame retardency, Tufban’s LOI value of 30-32% makes it an excellent fire resistant material. It is claimed that normal washing and dry –cleaning do not effect its flame retardant characteristics.
Material | LOI(%) |
Tufban | 30-32 |
Cotton | 18-20 |
Polyester | 20-22 |
Acryllic | 19-20 |
Nylon | 20-22 |
10. Incorpation of carbon in viscose fiber
(a) Incorporation of carbon black for antistatic properties-Electrically conductive carbon is used for the production of electrically conductive viscose fibre. The conductivity increases with decreasing particle size of carbon. For the production of electrically conductive viscose fibres, a slightly alkaline , electric conductive carbon black with a particle size of 20 nm is dispersed in water and mixed in viscose solution prior to spinning. It is found that a loss of about 50% in tenacity of this fibre compared with the tenacity of regular viscose fibre. On the other hand electricity conductive is increased by five orders of magnitude.
(b) Incorporation of activated carbon-Activated carbon containing fibre with outstanding adsorption properties. For the production of activated carbon-containing viscose fibres, activated carbon from coconut with a very fine pores is used .This activated carbon is has to be aground to a very fine particle size and thoroughly dispersed in water in order to be incorporated in viscose fibre in a satisfactory way. These fibres are used in application in such as protective clothing against gases, flat structure for industrial gas adsorportion, shoe insoles odour-absorbing and antimicrobial wound dressing.
(c) Incorporation of graphite-Incorporating 40% of lubricating graphite with a purity 99.5% into viscose yields fibres with excellent lubricating properties which as packing and sealing for crankshafts. Packings from graphite containing viscose fibres may be used to a temperature of 180-2000c.They are stable in the Ph range 5-9 and predominantly used to seal pumps and fitting for water, salt solutions, weak organic and inorganic acids.
11. Alternative to the viscose process
The viscose process provides inexpensive route to regenerate cellulose in fibrous form it constituent a health hazard due to toxic pollutants. Two major route which are much easier and less polluting than the old viscose process.
 |
2. That produces a derivative from which cellulose can be easily generated for example-liquid ammonia salt system.
Organic solvent (Direct solvent)
1. Ammonia-ammonium thiocyanate- The solvent is prepared by condensing NH3 to a predetermined weight with a known amount of NH4SCN. Solution of cellulose in NH3-NH4SCN are prepared by making a slurry of cellulose and solvent and stirring at- 10 0c.Fibres have been spun using wet spinning ,dry spinning and dry-jet wet spinning containing 14% cellulose at a solvent composition of 24.5:75.5 (wt%) NH3:NH4SCN
2. N-Methylmorpholine N-Oxide and Water-
- It is the best solvent to dissolve viscose fibre.
- Its having strong oxidant property due to which it can dissolve cellulose.
- During the dissolving step time and temp. are maintained properly otherwise thermal degradation takes place. Generally temp. is around 1300c
- If the temp. is goes above 150 0 c ,DP of cellulose goes down for this we add some phenolic oxidant .It stabilize the solution and finally oxidize the colour compound.
 |
- It is another solvent which dissolve cellulose. It directly linked with very high reproducible.
- It was observed that fibres from wet spinning process exhibited superior properties.
- It makes an complex with OH-group of cellulose & help to dissolve cellulose.
 |
- Take mixture of DMAc and dried cellulose. The mixture is distilled at 1650c for 30 min. in N2 atmosphere
- Mixture is then cool down at 100 0c can add required amount of LiCl stirred at 80 0 c for 40 min.
- Generally this solvent can dissolve upto 15% (w/w) for DP-130 & 4% (w/w) for DP-1700.
Salt that melt at temp. below 1000c called ionic liquid. It can be used as green solvent. Or reaction media. Generally contain imidazolium, pyridinium or organic ammonium salt. The anions could be chloride, bromide. Room temp. have more complex structure .The polymer can be regenerated by precipitation with water. They are able to achieve a very high polymer concentration of up to 25%.
 |
Two component system (amine salt) consisting of hydrazine (NH2-NH2) or ethylenediamine (NH2-CH2-CH2-NH2) and various thiocyanate salt such as LISCN,NASCN or KSCN that dissolve cellulose pump. However a high concentration 40-50% . Salt is generally required to obtain high concentration (upto 18-20% w/w) spinning dope.
 |
property | cotton | Ordinary viscose | HWM | polynosic |
Average Dp | 1600-2000 | 300 | 400 | 500 |
Dry tear strength(cn/tex) | 22 | 22 | 35 | 38 |
Wet tear strength(cn/tex) | 28 | 12 | 20 | 30 |
Water retention(%) | 50 | 90-100 | 75 | 50-70 |
Degree of fibrillation | 2 | 1 | 1 | 3 |
 |
Difficulties
- Old batch-wise process to continuous or semi-continuous system. In the batch wise process the sequence of steeping ,pressing and ageing took up to 40 hr. to produce alkali cellulose.
- Difficulties in ensuring contact temp. and equal ageing time, because a large no. of bins involved ,frequently resulted in a variable degree of polymerization in the resultant viscose.
- In the modern plants bales of wood pulp are automatically fed into continuously sulrry.
- By the use of catalyst and elevated temp during ageing have reduced to time 4-5 hr.
- Xanthation process has been improved with the use of wet churns. In which both xanthation and mixing carried out.
- More recently the introduction of back flush filters with non-woven metal screens has improved the filtration efficiency with the new non- woven metal screens the filtration amount has increased 50 fold. Thus the filtration size could be decreased.
- Completely automatization.
Reduction in chemical used such as CS2,NaOH and H2SO4. There are number of ways to achieve reduction in the amount of CS2 used in viscose & also allow a substantial reduction in viscose alkali content. Process-SINI process-Its also known as double steeping process operation of aged alkali cellulose at lower alkali concentration (10-12%).A second steeping after ages reduces the amount of free alkali in the crumb with out changing the bound alkali. This reduces the formation of by-product and improves distribution of xanthate group to get a stable viscose.
Advantages
- This process yield a 30% reduction in CS2 usuage, reducing CS2 emission.
- It is claimed that even interior-grade pulp can be used with this process to yield a good quality of viscose fibre.
- Due to removal of low molecular weight fractions. In the second steeping as well as increased rate of swelling of alkali cellulose which increases the reactivity to CS2 during xanthation .With this process a higher CS2:NaOH ratio (9:4.5) can be used in viscose solution which result in a substantial reduction in H2SO4.
- Activation of cellulose with liquid ammonia prior to xanthation also reduces CS2 consumption by as much as 33%.
- Xanthation in the presence of surfactants like Berol spin decreases CS2 consumption without effecting the quality of rayon produced.The addition of urea to the steeping solution result in change viscosity of viscose, the ripening time decreases and a high degree of xanthate substitution is obtained.It is presumed that complex with the alkali cellulose is formed which control the side reactions occurring during xanthation process.
- A reduction in viscosity of viscose allow for an increases in α-cellulose content & leads to reduction in consumption of H2SO4 & also less amount of energy is required for transport, filtration, deaeration.
13. Conclusion
- Development in process technology & process chemistry are much environment friendly
- Polynosic fibre show high crystallinity ,high resistant ,high dimension stability.
- By different solvent ,spinning specifications ,modifiers . We can make end use product.
- Solvent is very costly so need to recycle it.
- Ethylenediamine/NaSCN System is the best solvent
- Spinning of cellulose from N-methyl morpholine N-oxide in the presence of additives, polymer 1990,vol 31,march.
- Structure formation of regenerated cellulose materials from NMMO solutions, progress in polymer science 26(2001). 3. Novel cellulose solvent system and dry jet wet spinning of cellulose ED/KSCN solution, by hyun jik cel (thesis)
Our trained chemists work on custom projects designed specifically for the needs of each client. 1-butyl-3-methylimidazolium tricyanomethane
ReplyDelete